Coaxing cancer cells to commit suicide
4 Dec 2020
Chemical inhibition of an enzyme that is essential for DNA repair offers a possible strategy for the treatment of specific cancers.
4 Dec 2020
Chemical inhibition of an enzyme that is essential for DNA repair offers a possible strategy for the treatment of specific cancers.
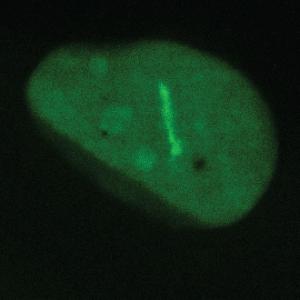
The intensive staining shows cells with a deficient DNA repair machinery | © Ladurner Group
In Germany, as in most Western countries, cancer ranks second to cardiovascular disease as the most frequent cause of death. Although the term ‘cancer’ covers a wide range of disorders that vary significantly in their course and frequency, all cancer syndromes have one feature in common: Unlike normal cells, cancer cells proliferate in an uncontrolled fashion.
Loss of the ability to regulate cell growth and division often is associated with deleterious mutations in the DNA, the hereditary material, which determines the molecular composition, and therefore the functions, of all cell types in the body. Among the agents that can cause such mutations are ultraviolet light, X-rays and gamma rays, as well as specific chemicals, toxins and highly reactive forms of oxygen known as oxygen radicals. In addition, hereditary (‘inborn’) mutations can also promote the development of cancers. Since mutagens are ubiquitous, and mutations frequently occur during DNA replication, cells have evolved multiple mechanisms that repair damaged DNA. Depending on the size and nature of the lesion, cells activate diverse responses to DNA damage. Repair enzymes are able to correct small-scale defects in cellular DNA, while cumulative and irreversible lesions trigger apoptosis – a form of induced cell suicide.
The mechanisms that mediate DNA repair are vital for survival. However, since tumor cells divide faster than many other cell types, agents that actively promote DNA damage are often used as therapeutic agents. This approach selectively eliminates cancer cells that have already sustained a significant degree of DNA damage. In this particular context, functional DNA repair systems are a hindrance rather than a help. This explains why researchers have long sought to identify weak points in the network of repair pathways that could serve as drug targets for the treatment of cancers – and the search has not been in vain.
One such target is a class of enzymes known as poly(ADP-ribose) polymerases (PARPs, for short), of which 18 have been identified in humans. “PARP1 and PARP2 are DNA repair enzymes, whose mode of action the field has been studying for 50 years now,” says Professor Andreas Ladurner, who holds the Chair of Physiological Chemistry at LMU’s Biomedical Center. “Especially since 2005 PARPs have been intensively studied as potential drug targets in the context of cancer” he adds.
PARP inhibitors have been particularly useful in the treatment of breast and ovarian cancers. In many cases, these cancers lack functional BRCA1 or BRCA2 genes. The proteins encoded by these genes are involved in the repair of single-stranded breaks in DNA. Loss of these genes disables this particular pathway, and forces the tumor cells to use an alternative mechanism in which PARP enzymes are key. This dependence on PARPs makes them highly vulnerable to drugs that inhibit these enzymes.
Although PARP inhibitors are clinically effective, they are not free of side-effects – partly because they interfere with the functions of PARP enzymes beyond DNA repair. They are therefore not suitable for use in all patients.
Ladurner and his colleagues hope to change this, and have begun to search for alternative points of attack. In their latest study, they set out to answer two questions: Aside from PARPs, what other factors are required for the repair of DNA damage, and which of these might provide promising targets for therapeutic drugs?
“PARP1 and PARP2 act like a trumpet. Once they bind the damaged DNA, they send out a strong DNA damage signal that promotes repair responses; they are not directly involved in the execution of DNA repair,” Ladurner explains. “This is where another enzyme, called ALC1, comes into play, as it specifically responds to the signal sent out by the PARPs.” To identify the precise function of ALC1, he and his team used genetic methods to create cells that lacked the functional ALC1 protein. A closer look at the properties of these cells revealed that, in the absence of ALC1, PARP enzymes remain bound to the site of DNA damage.
“Interestingly, this ‘trapping’ effect is comparable to that caused by inhibitors of PARPs 1 and 2,” says Ladurner. But while the PARP inhibitors currently in use block the generation of the signal produced by the PARPs, inactivation of ALC1 disables the DNA repair machinery itself. This novel approach therefore provides a more direct means of inhibiting the repair pathway – and leads to the death of tumor cells without disrupting the function of other PARPs. Indeed, loss of ALC1 function potentiates the action of PARP 1 and 2 inhibitors.
Ladurner hopes to translate laboratory findings like this into new therapies. He is a co-founder of Eisbach Bio, a start-up located in Planegg near Munich. He and his colleagues have already identified drug candidates for the inhibition of targets like ALC1 in tumors, which they hope to test in humans in the near future.
Molecular Cell 2020