Diabetes research: A new model for studies of beta-cell function
10 Sept 2021
Scientists have developed a genetically modified pig model for the age-dependent in-vivo labeling of insulin granules.
10 Sept 2021
Scientists have developed a genetically modified pig model for the age-dependent in-vivo labeling of insulin granules.
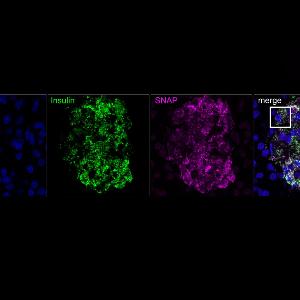
Source: Kemter et al., PNAS 2021
The beta cells of the pancreas synthesize, store and secrete the hormone insulin, which controls the level of glucose in the blood. Perturbation of the function of these cells is a major cause of adult-onset Type-2 diabetes, a condition that affects several million people around the world. Each beta cell contains thousands of organelles, known as secretory granules (SG), which release insulin into the bloodstream when the level of blood sugar rises. However, only a small fraction of the granules in each cell is released – which preferentially consists of those that were most recently synthesized. The mechanisms responsible for this process are not fully understood. In addition, our current knowledge of beta-cell function is largely based on studies on ex-vivo cell clusters (islets) isolated from rodents. In order to close the translational gap between these model systems and humans, scientists led by Eckhard Wolf, who holds the Chair of Molecular Animal Breeding and Biotechnology at LMU, together with researchers based at the Paul Langerhans Institute of the Helmholtz Zentrum München at the University Hospital Carl Gustav Carus and Faculty of Medicine of TU Dresden (PLID), have developed a transgenic pig model, which makes it possible for the first time to reproduce the pattern of insulin turnover seen in humans when levels of blood sugar are within the normal range.
The team began by inserting what is called a SNAP-tag into the pig gene for insulin, which enabled the protein to be labeled with different fluorescent dyes over long periods of time. “Next, we had to transfect this vector into pig cells. These were used for somatic cell nuclear transfer, to generate pigs that expressed the tagged gene in their beta cells,” explains PD Dr. Elisabeth Kemter, a member of Wolf’s group, and joint first author of the study. The animals with the highest levels of expression of the SNAP-tag in their beta cells – referred to as SOFIA (Study OF Insulin granule Aging) pigs – were then characterized in further experiments.
“Overall, the ultrastructure of the beta cells of SOFIA pigs was comparable to that of pigs not expressing insulin-SNAP, and the insulin SGs, mitochondria and endoplasmic reticulum appeared normal without any signs of stress or structural alterations,” says Dr. Andreas Müller, a member of Michele Solimena’s team at PLID and also joint author of the report. Furthermore, both glucose homeostasis and insulin secretion are normal in SOFIA pigs.
The researchers were able to show that the sequential in-vivo addition of two SNAP-tag ligands that fluoresce at different wavelengths enabled them to distinguish different pools of insulin secretory granules in the beta cells of SOFIA pigs from each other – on the basis of their color. This in turn enabled them to follow the rates of turnover of the secretory granules under essentially natural conditions. It emerged that the turnover times of both recently synthesized and older secretory granules were significantly shorter in vivo than those obtained for rodent pancreatic islets that were cultured in vitro.
The authors of the study are convinced that the successful generation and characterization of the new experimental model represent an important step on the way to overcoming the limitations that currently hamper beta-cell research. SOFIA pigs can now be crossed with other pig strains that serve as model systems for the study of diabetes mellitus, in order to determine the rates of synthesis and turnover of insulin secretory granules under pathological conditions.
Elisabeth Kemter, Andreas Müller, Martin Neukam, Anna Ivanova, Nikolai Klymiuk, Simone Renner, Kaiyuan Yang, Johannes Broichhagen, Mayuko Kurome, Valeri Zakhartchenko, Barbara Kessler, Klaus-Peter Knoch, Marc Bickle, Barbara Ludwig, Kai Johnsson, Heiko Lickert, Thomas Kurth, Eckhard Wolf, Michele Solimena: Sequential in vivo labeling of secretory granule pools in INS-SNAP transgenic pigs. PNAS 2021