Hearts and minds
29 Nov 2023
Pigs are used as model organisms for studying diseases, and they could also become organ donors for humans. For our science magazine EINSICHTEN we visit LMU’s Center for Innovative Medical Models (CiMM) to find out more.
29 Nov 2023
Pigs are used as model organisms for studying diseases, and they could also become organ donors for humans. For our science magazine EINSICHTEN we visit LMU’s Center for Innovative Medical Models (CiMM) to find out more.
The great hope of transplantation medicine has a shaggy appearance – and a curious nature. Its little black eyes scrutinize every new arrival, its snout cocking up and grunting at them. With its bristly coat and stocky build, the Auckland Island Pig somewhat resembles a wild boar. In any event, it is a breed with a special trait: “The heart of a fully grown animal is roughly the same size as that of an adult human,” explains veterinarian and geneticist Eckhard Wolf, as he guides us through the pigsties at LMU’s Center for Innovative Medical Models (CiMM) in Oberschleißheim. As such, the breed could be a godsend for biomedicine. In the future, the Auckland Island Pig could be used as an organ donor for humans. Certainly, there is no shortage of demand for organs. Some 8,500 people in Germany alone are on waiting lists for an organ transplant, while last year there were only around 850 donations of human organs.
In the rustic environment of the sties, stringent hygiene is the order of the day. The experimental pigs must not be infected with any pathogens. Anyone who wants to access the sties – whether researcher, carer, or visitor – must first pass through a hygiene lock. Off with shoes and socks, disinfect hands and feet, into the shower – “Don’t forget to wash your hair!” instructs Wolf – and then on with disposable underwear and freshly washed clothes. Only when you are washed from head to toe are you permitted to access the animals.
In the nearest pen, seven rosy piglets loll next to their mother under a heat lamp. “They’ve just filled their bellies,” says Wolf, also freshly showered, while he takes photos of the animals with his phone for his private collection. “In about an hour, the suckling will begin all over again,” he observes.

Eckhard Wolf, veterinary surgeon and genetic researcher, manages the Center for Innovative Medical Models (CiMM) near Oberschleißheim. | © Christoph Olesinski / LMU
Around 500 pigs in total are housed in the high-tech building near Munich, which is roughly 80 meters long and 40 meters wide. About a third are used for transplantation research, while the remainder serve as model organisms for genetic disorders or the chronic metabolic disease, diabetes. “Animal models are essential for investigating disease mechanisms and testing new diagnostic methods and therapies,” explains Wolf. Pigs are currently the best candidates for these studies, because they resemble humans in their anatomy and metabolism.
Animal models are essential for investigating disease mechanisms and testing new diagnostic methods and therapies.Eckhard Wolf

Read the latest findings on holding things together in the current issue of our research magazine EINSICHTEN at www.lmu.de/einsichten. | © LMU
Whether destined for organ donation or for use as animal models, the pigs are genetically modified. Some of them, for example, have a mutation in a specific gene that leads to the genetic disease called Duchenne muscular dystrophy. This disease causes muscles to degenerate, resulting ultimately in death. In other pigs, their genetic material is modified so that they develop fewer insulin-producing cells in the pancreas than normal. As a result, they are less well able to process glucose – just like people who suffer from diabetes. Others again are genetically engineered such that specific structures of their insulin-producing cells are tagged with a fluorescent molecule. “In this way, we can trace the insulin turnover in a living organism,” says Wolf as he points at the animals in question, which look like perfectly ordinary domestic pigs: They grunt, sniff, squeal – and smell … well, like pigs.
Let us return to the Auckland Island Pigs, whose history begins at the start of the 19th century when European pigs were first released on the Auckland Islands (465 kilometers south of New Zealand) in 1807 by Abraham Bristow, followed by at least two other releases until the mid-19th century. The new arrivals formed a small breeding population. In 1999, the Rare Breeds Conservation Society of New Zealand removed 17 of the feral pigs from the main Auckland Island and established a small herd under designated pathogen-free conditions, from which the Auckland Island pigs I Wolf’s facility are derived.
But why do their genes have to be modified to make them suitable for potential organ donation? Wolf explains: “On the surface of pig cells, there are certain sugar molecules which humans have antibodies against. Now if we were to implant pig cells or entire pig organs into humans, these antibodies would bind to the sugar structures and activate the body’s defense mechanisms.” This response would quickly destroy the pig cells and thus the entire organ.
Organ rejection is one of the biggest challenges facing transplantation medicine – and that goes especially for xenotransplantation, the technical term for transplanting organs between different species. The body does not tolerate any intruders and fights them tooth and nail – including transplanted organs. That being said, the reaction is weaker according to the similarity between the donor and recipient, meaning that human organs are most suitable for transplants. Unfortunately, however, demand far outstrips supply.
A lot of hope is invested therefore in pigs – and in genetic engineering: “We inactivate the genes that are responsible for synthesizing the sugar residues,” explains Wolf. In addition, the animal genome is modified such that it inhibits the human rejection response. “Another problem is that clotting can occur when human blood flows through porcine blood vessels,” says Wolf. Therefore, the pig cells are genetically engineered to contain human proteins that regulate the blood clotting.
Healthy people may view the notion of living with a pig heart as outlandish. But for some people, it could be their only chance of survival.Eckhard Wolf
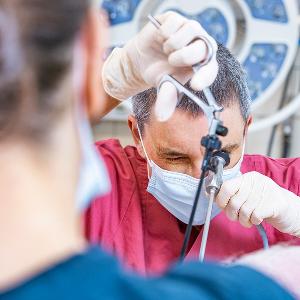
Veterinarian Barbara Kessler implants a genetically modified embryo into the oviduct of a pig. | © Christoph Olesinski / LMU
Next to the sties, there is an old farmhouse – the whole setting feels more like a bucolic farmstead than a modern research facility. But looks are deceiving: The former pens house state-of-the-art biotechnology labs, where countless tissue and blood samples are stored in large freezers. The farm belongs to LMU since 30 years. Eckhard Wolf has been here since the beginning and has headed the LMU facility for the past 28 years.
In the laboratories on the ground floor of the farmhouse, Wolf’s staff begin by modifying the genome of an individual pig cell. One of the tools they use to do this is the gene scissors CRISPR/Cas9, a molecular scalpel with which they are able to precisely cleave the DNA and remove gene segments or add new ones. Next, the researchers implant the genetically modified cell nucleus into an enucleated egg cell, a technique that was pioneered in 1997 when cloning the famous sheep Dolly.
“We successfully reproduced the experiment shortly afterward,” recounts Wolf, proudly showing the workstation at which the groundbreaking experiment was done. But back to the method: “The prepared egg cells grow into embryos, which are then implanted into the oviducts of a sow,” continues the cloning expert. If everything works as planned, piglets containing a targeted genetic modification are born a few months later. These animals can then multiply in the normal way through breeding.
How advanced is Wolf with his work? When will it be possible to implant the pig hearts in humans? “I think we’ll have the right donor animals in two years,” says Wolf – that is to say, ones that contain all the planned genetic modifications. The hearts of these pigs must then be tested on baboons. “If this transplantation is successful, we can apply for an initial clinical pilot study with four to six patients.”
In previous experiments which implanted hearts from genetically modified landrace pigs into baboons, the monkeys survived for about six months. However, the experiments, which were carried out within a Collaborative Research Centre funded by the German Research Foundation (DFG), also showed that the young pig hearts continued to grow after the transplant as if they were still in the pig. As a result, the hearts became too large at some point for the ribcage of the monkeys, which weigh around 20 kilograms. Although the growth can be restricted through genetic engineering, the more genetic modifications you have in different chromosomes, the harder it becomes to produce the right combination in the end through breeding. “For this reason, we opted for the smaller Auckland Island Pigs, which have a smaller heart,” says Wolf.
I think that we will have the right donor animals in two years' time.Eckhard Wolf
In Wolf’s opinion, then, the size and rejection responses are solvable problems. On top of this, it must be ensured before transplantation that the animal donor heart is not infected with viruses. This was likely what sealed the fate of the first xenotransplant patient David Bennett. On 10 January 2022, the American was the first ever person to be implanted with a genetically modified pig heart.
Bennett suffered from end-stage heart failure, accompanied by cardiac arrythmias. On account of the advanced state of his disease and because he did not sufficiently adhere to the prescribed therapeutic measures, he was considered ineligible for a human donor heart. The pig organ was therefore his only chance. Two months after the operation, Bennett died – probably due to swine viruses. “By means of a simple antibody test on the donor animal, such an infection could have been discovered in advance,” says Wolf. “But Bennett’s state of health was exceptionally poor to begin with. Presumably, a xenotransplantation wouldn’t have been able to save him in the long run in any event.”
Was the transplantation nevertheless a success in Wolf’s view? “It was,” he admits, “a hazardous experiment” as regards the patient’s chances of survival, even if we ignore avoidable complications. The risk of a backlash for the entire research surrounding xenotransplantation was also undoubtedly great. However, the experiment demonstrated, according to Wolf, that the approach can work – even in a patient whose existing circumstances were anything but ideal.
So, for which patient groups would such surgery be a justifiable option? Wolf defines an ‘ideal’ patients as follows: “It’s someone for whom we can expect a positive effect on their health. And at the same time, who is not eligible for a human heart or mechanical support systems.” As examples, he points to cancer patients, who do not receive a human organ because of their high risk of death; and infants with serious heart defects, an age group for which there are very few donor hearts available.
“Healthy people may view the notion of living with a pig heart as outlandish,” says Wolf. “But for some people, it could be their only chance of survival.”
And so pig hearts could become a business just like other medical developments, because donor organs are so scarce. In that case, the breeding of the animals would no longer be in the hands of research institutions, but of private enterprises. “The costs of a pig heart transplant will be determined by mechanical heart support systems, so roughly in the range of 80,000 to 150,000 euros,” estimates Wolf.
And hearts will not be the only organs that could be transplanted. “Theoretically, our heart donor pigs would also be suitable as kidney donors,” says Wolf. Furthermore, researchers at the Center for Innovative medical Models are already working on pigs that are potential donors of pancreatic islets for the treatment of type 1 diabetes. Pancreatic islets are groups of cells in the pancreas that register blood sugar levels and produce insulin. In patients with type 1 diabetes, these islets do not work properly.
There is de facto no drug that has found its way into clinical practice without animal experiments.Eckhard Wolf
These are the conceivable xenotransplants. “But many more patients,” stresses Wolf, “will ultimately benefit from model organism research.” Only with the aid of such experiments can new therapeutic and diagnostic approaches be put in the hands of doctors. As an example, Wolf references experiments on animals with Duchenne muscular dystrophy (DMD), by which the researchers tested a new imaging diagnostic technique. The non-invasive approach could replace the six-minute walk test that doctors routinely use to monitor the progress of DMD and the effects of a treatment. With the new method, the result is not dependent on the motivation of the patients and their remaining ability to walk.
For all the research successes, what about the criticisms of animal rights advocates? The veterinarian says he rarely hears such complaints. After all, the facility has nothing to hide and is very transparent. “Of course, we must never ever do a single unnecessary animal experiment – and experiments must always be carried out by experts with the proper training.” That is the only way to ensure that unnecessary suffering is truly avoided, he notes. Since 2011, moreover, the German Ethics Council has called for – as it does for all animal experiments – “the keeping of the test animals in species-appropriate conditions and the avoidance of pain resulting from the implantation of a human gene.” The ethics board, which is the authority in such matters, has no objection in principle to genetic engineering on animals for experimental purposes.
Wolf specifies his approach here: “If a pig saves the life of a human, then the pig’s life has been used well.” Not everybody agrees with this view. The organization Doctors Against Animal Experiments, for example, is fundamentally against animal experiments in any form. “There is de facto no drug that has found its way into clinical practice without animal experiments,” rejoins Wolf. And finally there is the question of proportionality: “For decades, around fifty million pigs have been slaughtered in Germany every year for meat production, whereas just a few thousand have been used for important experimental purposes over the same period,” argues Wolf.
Compared to the conditions in large-scale livestock farming, the pigs at the animal testing facility have a lot of space and are able to freely move around in their group, as Wolf demonstrates to us. They are not, however, allowed outside. The pigsties are fully closed off and additionally surrounded by an extensive fence, so that wild boars cannot get too close to the building. Out in the open, there is always the risk of the pigs becoming infected with pathogens. And that would be devastating, in the worst case wiping out the investment and hard work of decades.
Text: Janosch Deeg
Prof. Dr. med. vet. Eckhard Wolf is Chair of Molecular Animal Breeding and Biotechnology at the Gene Center Munich and the Department of Veterinary Sciences at LMU. Born in 1963, Wolf studied veterinary medicine at LMU, where he also completed his doctorate. He worked as a postdoc first at the Institute of Animal Breeding at LMU, and then as the leader of a research group at the University of Veterinary Medicine, Vienna. It was in Vienna that he obtained his habilitation degree, before returning to his alma mater in Munich as a professor in 1995. Wolf is Director of the Center for Innovative Medical Models (CiMM) at LMU and spokesperson of the DFG-funded Collaborative Research Centre “Biology of Xenogeneic Cell and Organ Transplantation – From Bench to Bedside.”
Read more articles from "EINSICHTEN. Das Forschungsmagazin" in the online section and never miss an issue by activating the e-paper alert.
Or subscribe to the free print version of EINSICHTEN (in german).