Novel spatial-omics technology enables investigation of diseases at their early stages
22 Dec 2022
Researchers have developed a new method that uses robotics technology to obtain proteomics data from cells.
22 Dec 2022
Researchers have developed a new method that uses robotics technology to obtain proteomics data from cells.
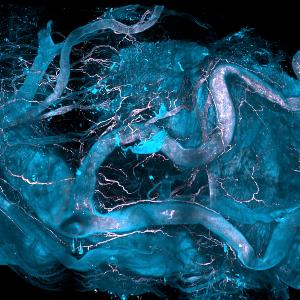
Aortic regions of a human heart whose vascular plaques are analyzed by DISCO-MS technology. | © Helmholtz Munich, Harsharan Singh Bhatia und Ali Ertürk
How can you trace a single diseased cell in an intact brain or a human heart? The search resembles looking for a needle in a haystack. The teams of Ali Ertürk at Helmholtz Munich and LMU Munich and Matthias Mann at the Max Planck Institute of Biochemistry in Martinsried near Munich have now developed a new technology named DISCO-MS that solves the problem. DISCO-MS uses robotics technology to obtain proteomics data from ‘sick’ cells precisely identified early in the disease.
Most diseases are asympotomatic initially and the affected persons usually still feel fine - symptoms are not yet present, or still too mild to realize. However, a change has already happened within the body: a virus may have started replicating, or a rogue cell might have divided more often than it should have. But how can these changes be perceived?
Researchers face a similar dilemma when they are studying the early development of diseases. Even when working with animal models, scientists can rarely pinpoint the small sites of disease initiation or characterize the exact molecular changes that drive disease progression.
With the development of DISCO-MS by the research teams of Ertürk and Mann in Germany, this task has become much easier. DISCO-MS combines methods to turn mouse and human tissues transparent with the latest robotics and proteomics technologies to determine their molecular makeup.
DISCO-MS starts with the so-called DISCO tissue clearing, which renders the mouse body or human organs transparent - making them accessible to imaging. Thereby, fluorescently labeled cells can be readily identified in intact tissues of specific sites using high-resolution three-dimensional microscopy.
Once the regions of interest have been identified, they are isolated using a new robotics technology called DISCO-bot, developed by mechanical engineer Furkan Öztürk, a Ph.D. student in Ertürk’s lab. The robot-assisted extracted tissues are processed for their proteome analysis using advanced mass spectrometry (MS) methods developed by Andreas-David Brunner, a former Ph.D. student in Mann’s lab. This high-tech approach allows complete molecular characterization of any desired tissue region identified in 3D in whole mouse bodies or human organs.
To showcase the method’s power, first author Harsharan Singh Bhatia and colleagues applied DISCO-MS to Alzheimer’s disease (AD) mouse model and to atherosclerotic plaques (pathological hardening and narrowing of blood vessels) in the human heart. In the tissue samples of the AD model, the team applied artificial intelligence (AI) to identify the typical AD plaques at the early stages of the disease, which had been difficult to detect by any other method. Subsequent proteomics analyses of the plaques provided an unbiased and large scale study of proteins affected in AD, revealing new molecular players that could be biomarkers for Alzheimer’s disease.
In the human heart, the researchers were interested in the composition of the tissues around atherosclerotic plaques, which were quickly visible after tissue clearing. AI detection and robotics extraction of the tissues again allowed the identification of dysregulated molecular pathways in human heart cells related to aortic plaques. These results are key findings, as they form the basis for potential therapeutic targets.
DISCO-MS is the first spatial-omics technology in intact 3D volumes and accelerates studying complicated diseases ranging from cancer to metabolic disorders. As DISCO-MS works with pre-clinical and clinical tissues, it enables the study of diseases at their earliest stages and, subsequently, the development of potential new therapeutics. Source: Helmholtz Munich/LMU
Bhatia et al.: Spatial proteomics in three-dimensional intact specimens. Cell 2022