Regulation of cell division by the signaling protein ECT2
2 Mar 2021
During chromosome segregation, the dividing cell changes its shape so that two daughter cells are formed. This latter step is coordinated by the protein ECT2.
2 Mar 2021
During chromosome segregation, the dividing cell changes its shape so that two daughter cells are formed. This latter step is coordinated by the protein ECT2.
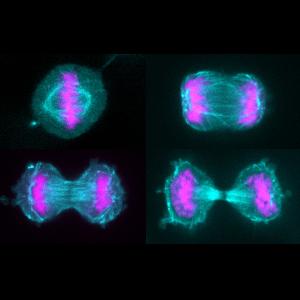
Cultured human HeLa cells during cell division. The spindle apparatus is shown in cyan, the chromosomes in pink. | © Friederike Wolff, Zanin Group
During the development and lifetime of multicellular organisms, cells continuously divide millions of times. In the course of each and every cell division, the genetic information of the mother cell must be duplicated and then distributed equally to the two daughter cells. This process enables embryos to grow, and tissues to be repaired and remodeled.
Cell division, also known as mitosis, is a highly complex and multistage process. It begins with the duplication of the genetic material called DNA stored in the cell nucleus. Subsequently the replicated DNA molecules interact with specific proteins, which causes them to condense into small threads called chromosomes. Chromosome condensation marks the onset of the first stage of cell division. This is followed by the disassembly of the nuclear membrane, and the assembly of a structure made up of organized tubulin polymers. This structure, known as the spindle apparatus, positions the replicated chromosomes in the equatorial plane of the dividing cell. In the following stage (‘anaphase’), the spindle separates the chromosome pairs and transports them to opposite poles. This process ensures that the two sets of genetic material are correctly distributed between the daughter cells. When the chromosome complements have fully separated, a new nuclear membrane forms around each set. Finally, a equatorially located contractile ring constricts the dividing cell – giving rise to two daughter cells, each of which contains a complete copy of the genome of the mother cell.
The research group led by Esther Zanin at the LMU Biocenter is primarily interested in this last step – the division of the mother cell into two daughter cells, which is called cytokinesis. “Defects in this phase can lead to congenital diseases and cancer,” Zanin points out. “Our work focuses on teasing apart the molecular mechanisms that regulate cytokinesis in space and time.”
During cytokinesis, the contractile ring that forms along the equator of the dividing cell acts like a purse string, gradually constricting the dividing cell to produce two daughter cells. The ring itself is made up of actin and myosin, two filament forming proteins that also mediate muscle contraction. Formation of the actin-myosin ring during cell division must be temporally and spatially coordinated with the progression of mitosis and the segregation of the chromosomes to the daughter cells, respectively.
“The spatial and temporal control of cell division is very important, because it is essential to ensure that the division of the mother cell is initiated only after the chromosomes have segregated to opposite poles,” Zanin explains. Previous studies had shown that a specific signaling protein called ECT2 triggers formation of the contractile ring, but how ECT2 activity is regulated remained unclear.
ECT2 is a large protein, which consists of several structural domains. These domains are thought to be involved in mediating the activation and inhibition of ECT2’s function. Zanin and her colleagues set out to test this hypothesis.
To do so, they engineered ECT2 mutant proteins in which single domains were deleted in cultured human cells, and examined their effects on the assembly of the contractile ring and cytokinesis. “It turned out that different domains of ECT2 have opposite effects on its regulation,” Zanin says. Biochemical studies also revealed that different domains of ECT2 bind to and are modified by distinct signaling molecules. Together, these interactions and modifications most probably serve to integrate different signals. Overall, the results indicate that regulation of ECT2 is more complicated than was previously envisaged.
Thus, many questions remain unanswered. “We plan to pursue this topic further,” says Zanin. The next step is to understand how the binding of the various signal molecules to the different domains of ECT2 either increases or reduces its activity. In addition, earlier findings showed that ECT2 is often mutated in tumor tissues. “Whether this is a causative effect or a by-product of the malignancy itself is not yet clear,” says Zanin – and this issue also opens avenues for future research.