Shift change: sleep and the immune system
12 Jul 2024
When the lights are out and we’re fast asleep, our immune system is busy at work. Luciana Besedovsky wants to explain the underlying mechanisms. From the research magazine EINSICHTEN
12 Jul 2024
When the lights are out and we’re fast asleep, our immune system is busy at work. Luciana Besedovsky wants to explain the underlying mechanisms. From the research magazine EINSICHTEN
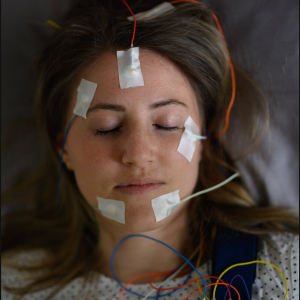
A test person spends the night in the sleep laboratory with EEG electrodes attached to her head. | © Alessandra Schellnegger / LMU
“Are you sleeping well?” We’ve all been asked this question at one time or another, perhaps by our family doctor. Healthy sleep is clearly important – but why is this? Our nightly slumber is much more than a standby mode in which our body is suspended until it gets bright outside. “Sleep has a comprehensive influence on our health and our immune system,” says Luciana Besedovsky. People who habitually sleep too little have a higher risk of catching an infection. Sleep is even necessary for survival, as demonstrated by early animal studies from the 1980s, where researchers found that the body’s internal defense mechanisms broke down after several sleepless weeks. Besedovsky, who has headed the Sleep and Immunology research group at LMU since 2021, investigates such connections between sleep and the immune system in humans.
But what exactly happens in the body while we sleep? This question drives Luciana Besedovsky. “We might say that our immune system learns as we sleep,” says the scientist. Just like our brain embeds experiences in our long-term memory during sleep, the immune system memorizes defenses against previously unknown pathogens. Several studies have shown, for example, that a good night’s sleep after a vaccination has a positive effect on the resulting immunity. Test subjects who were allowed to sleep during the night after a vaccination formed around twice as many antibodies as the group who had to stay awake for 24 hours.
T cells play a major role here, forming a substantial part of the memory of our immune system. Once they come into contact with a pathogen, the T cells that recognize precisely this pathogen proliferate. In this way, they remember what the invader looks like. The next time a person is infected with the same germ, these so-called memory T cells are able to launch the defenses more quickly. Vaccinations are based on the same principle: harmless parts of a pathogen are presented to the T cells, thereby activating them and stimulating their division.
This process takes place in the lymph nodes. But the T cells do not always remain there. They also patrol the blood and get into various tissues in the search for invaders. When we sleep, some of the cells leave the bloodstream and migrate – so the hypothesis goes – to the lymph nodes. But if we don’t sleep, this migration is disrupted and more T cells remain in the blood.
We might say that our immune system learns as we sleep.Luciana Besedovsky
But do T cells really migrate to the lymph nodes during sleep, or do they go somewhere else in the body? During her time as a research group leader in Tübingen, Besedovsky investigated this question. She invited good sleepers who have no problems sleeping through the night into the laboratory. On two test days, the participants were monitored for 24 hours in the sleep lab. Every four hours, they gave a blood sample. At 11:00 p.m., the participants in one session were allowed to go to sleep, while in the other session, they had to stay awake in bed. During the night, the researchers took a blood sample even every hour. Thanks to a catheter connected to a tube running from the test subject to an anteroom through a thin hole in the wall, it was possible to take blood without waking them.
Analyses of immune cells in the blood revealed that T cells moved in the direction of the signaling molecule CCL19 to a greater extent when the participants were allowed to sleep. Because this molecule occurs primarily in the lymph nodes, this supports the hypothesis that the T cells actually do migrate there. This movement is mediated by the hormones prolactin and growth hormone. The LMU researcher and her team were also able to reproduce this hormone-dependent T cell migration in vitro. When the researchers blocked prolactin and growth hormone, it weakened the T cell migration.

Read more about highlights of research in the current issue of our LMU magazine EINSICHTEN at www.lmu.de/einsichten. | © LMU
This experiment illustrates one aspect of what happens with the immune cells in our body while we sleep. “But it’s not only a matter of whether and how much we sleep, but also of when,” explains Besedovsky. Ideally, we live in harmony with our biological clock. Sleep researchers distinguish between morning larks, who wake earlier and tire in the early evening, and night owls, who sleep for longer in the morning but who are fit and active into the night.
Luciana Besedovsky herself is a night owl. “I often used to have difficulties falling asleep,” she recalls. “When others were already fast asleep, I simply wasn’t tired enough.” In the morning, when the alarm clock rang, she felt as if she hadn’t got enough sleep. This chronotype is often unfairly labeled as lazy. “Getting up late doesn’t by itself mean somebody is lazy. The bodies of the various chronotypes just work differently,” says Besedovsky. However, this knowledge hasn’t seeped into society at large, and everyday life often does not accommodate these differences. Sleep experts, for example, have long called for secondary schools at least to start later, so that teenagers can get enough sleep.
“Wherever possible, people should live according to their internal clock,” argues Besedovsky. She has already put this principle into practice in her research group: While the morning larks come in at daybreak, the night owls arrive later and work into the evening. That is not only more pleasant, but also – according to the latest research – most likely healthier as well. “We were recently able to demonstrate that people with a late chronotype reported having a cold more often than people with a moderate chronotype,” says Besedovsky.
She also found similar results in people who suffer from “social jetlag” – that is to say, who sleep at different times on work days and days off, which is most common among late chronotypes. People with social jetlag also reported having a cold more often than people who go to bed at roughly the same time every night. “But whether that is really due to sleep behavior or other factors cannot be answered directly by means of such association studies. We want to investigate this subject in greater detail with our lab studies.”
But lab studies too have their limits. After all, it may be the case that people’s sleep is different in the strange environment of a sleep laboratory than in their familiar environments. Anyone who has ever slept worse on their first night in a hotel room than they do at home will know this effect. To further improve the validity of her sleep-and-immunology studies, the LMU scientist established sleep measurements including blood sampling at home: A headband with a built-in EEG measures the sleep phases. A prick in the fingertip is all that is needed to take the blood sample. Thanks to this set-up, experiments can now take place in people’s bedrooms at home.
Interestingly, it’s easier to find participants with slight sleeping issues than people with fully healthy sleep.Luciana Besedovsky
To date, Luciana Besedovsky has primarily studied good sleepers, but now she wants to look at people with mild sleep disorders as well. “Interestingly, it’s easier to find participants with slight sleeping issues than people with fully healthy sleep,” she reports. The number of people affected by sleep disorders has actually increased significantly – by a third over the past ten years alone, according to data from German health insurers.
Everyday life in modern society makes our daily rhythms more irregular and leads to a lack of movement. And the artificial light from bulbs and smartphone displays is an obstacle to come to rest. The same goes for the ever faster-flowing stream of information that floods our brains.
Good sleep has become rarer. Yet it is the key to our health. If someone’s biological clock does not tick in rhythm with the world, they can try to synchronize it a bit better with their environment by means of daylight. In concrete terms, that means getting out into the open air in the morning and switching off screens in the evening. And then: Sleep well!
Luciana Besedovsky has been a professor at LMU since 2021, where she heads the Laboratory for Sleep and Immunology at the Institute of Medical Psychology. Her research group focuses primarily on investigating how sleep affects human health and the immune system. Besedovsky obtained her doctorate at the Institute of Medical Psychology and Behavioural Neurobiology at the University of Tübingen, to which she returned as a group leader after a research residency at Harvard Medical School.
Read more articles from "EINSICHTEN. Das Forschungsmagazin" in the online section and never miss an issue by activating the magaziner alert.